Ch4 Polar Or Nonpolar - Is Ch4 Polar Page 1 Line 17qq Com - If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search.
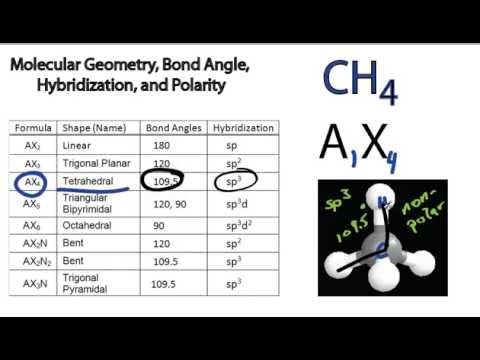
Ch4 Polar Or Nonpolar - Is Ch4 Polar Page 1 Line 17qq Com - If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search.. All formats available for pc, mac, ebook readers and other mobile devices. Nonpolar molecules with polar bonds. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. There are, however, c2h2 and ch4, both of which are nonpolar. Ch4 is nonpolar because all of the nonpolar covalent bonds are spaced within a tetrahedral structure around the molecule.
Is ch4 polar or nonpolar. Is brcl3 polar or nonpolar? Why is polar but nonpolar? Hey everyone, welcome to the mentor center! Nonpolar molecules with polar bonds.
Is brcl3 polar or nonpolar?
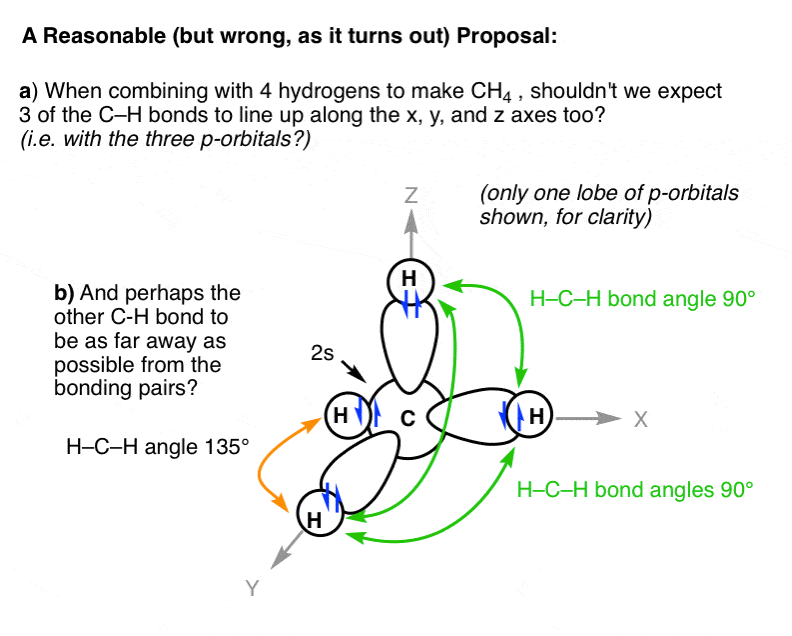
The shape for ch2cl2 is tetrahedral. List molecules polar and non polar. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. Is co2 polar or nonpolar? Submit answer retry entire gr. Is brcl3 polar or nonpolar? Polarity describes the distribution of electrical charge around a molecule. Is ch4 methane polar or nonpolar youtube , both molecular polar and ionic substances dissolve in water. Although the bonds themselves are polar, the four bonds between carbon and fluorine cancel out one another, generating a nonpolar molecule. The molecules that have atoms with equal electronegativity are nonpolar in nature because the equal charge why is ch4 nonpolar? No because water only dissolves polar covalent bonds and ch4 is nonpolar. Polar and nonpolar molecules are the two broad classes of molecules. In today's video, i determine whether methane (ch4) is polar or nonpolar.
Bir molekülün üzerindeki yük dağılımın simetrik olmaması demektir. I think a good way to solve these in general is to first draw the molecule and. Is ch4 polar or nonpolar. Nh3 is polar for the same reason as water. I'll tell you the polar or nonpolar list below.
There are, however, c2h2 and ch4, both of which are nonpolar.
Ch4 can constitute up to about 90 of natural gas depending on the the difference in electrostatic potential is also minimal giving an overall nonpolar molecule. Learn about polar nonpolar with free interactive flashcards. About solvents in organic chemistry. Methane ch 4 is a non polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms. Ch4 polar mı apolar mı? Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. Ch4 contains nonpolar covalent bonds because the electronegativity difference between hydrogen. Polar protic vs polar aprotic vs nonpolar: 3d shape of ch4 is tetrahedral where all the dipoles of the same two elements cancle out. Why is a molecule of #ch_4# nonpolar? Polarity describes the distribution of electrical charge around a molecule. Cf3cl in which c is the central atome, there are 4 bonding pairs around it. This distributes electron charge equally around the central carbon atom.
Polar protic vs polar aprotic vs nonpolar: Polar molecules vs nonpolar molecules. Is ch 4 polar or nonpolar? 3d shape of ch4 is tetrahedral where all the dipoles of the same two elements cancle out. I think a good way to solve these in general is to first draw the molecule and.
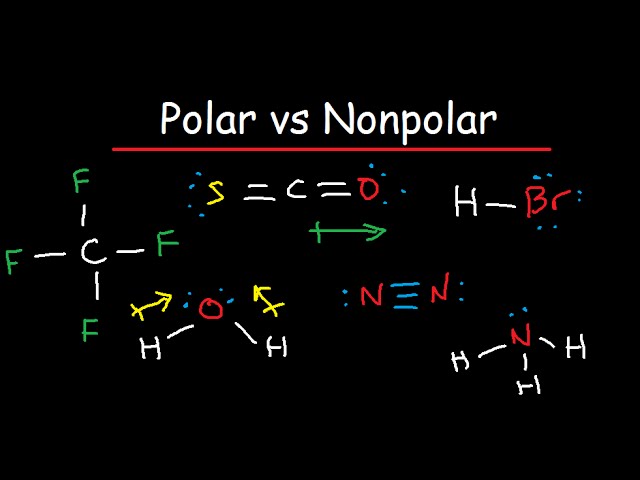
Polarity depends on the relative electronegativity values between two atoms forming a chemical bond.
Is ch4 methane polar or nonpolar youtube , both molecular polar and ionic substances dissolve in water. About solvents in organic chemistry. Is ch4 (methane) polar or nonpolar? If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search. Ch2 does not exist as a molecule. Learn about polar nonpolar with free interactive flashcards. The lewis structure drawing is really misleading because it makes you think that the chlorine atoms are directly opposite from each. Well, moreover, the polar solvents possess molecules with polar bonds, and nonpolar solvents possess molecules with similar electronegativity values. I think a good way to solve these in general is to first draw the molecule and. So, first off, methane (ch₄) is nonpolar because its c—h bonds do not have great enough of an electronegativity (e. List molecules polar and non polar. Ch4, or methane, is the same as cf4 in this respect, as are many other molecules made of four halogens surrounding a carbon or silicon atom. In order for a bond to be polar, it must have polar bonds, and the partial charges created by these polar bonds must not cancel.
Komentar
Posting Komentar